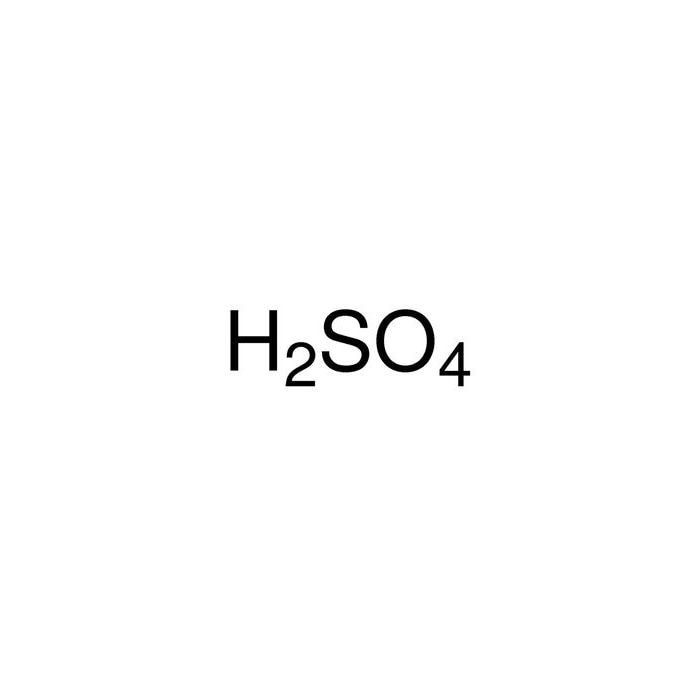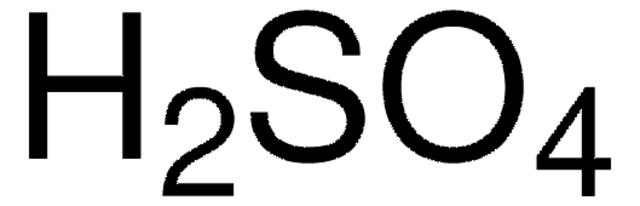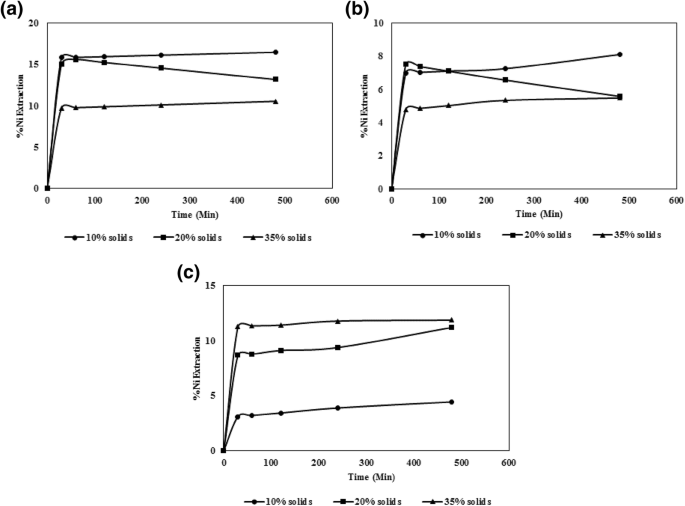
Characteristics of Leaching of Nickel from a Mafic Overburden in Sulfuric Acid and Sodium Chloride Medium at Atmospheric Pressure | SpringerLink
![SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM](https://cdn.numerade.com/ask_previews/8be369ab-e2f0-4054-a35e-d3e0c372f22f_large.jpg)
SOLVED: Fe(OH)3, Co(OH)3, Ni(OH)2, MnO2 REACTION WITH [H2SO4, H2O2] HOW TO GET Fe3+ Co2+ Ni2+ Mn2+ JUST WRITE NET IONIC EQUATION FOR 4 OF THEM

Kinetics of nickel leaching from low-nickel matte in sulfuric acid solution under atmospheric pressure - ScienceDirect

A Little Nickel Goes a Long Way: Ni Incorporation into Rh2P for Stable Bifunctional Electrocatalytic Water Splitting in Acidic Media | ACS Materials Au

Leaching Kinetics of Mo, Ni, and Al Oxides from Spent Nickel–Molybdenum Hydrodesulfurization Catalyst in H2SO4 Solution | SpringerLink

Comparison between experimental and calculated I-E curves. Cu-Ni in 0.5 M H2SO4 + 10 −4 M cysteine: cathodic scan (cf. Fig. 2).

Synergistic Recovery of Valuable Metals from Spent Nickel–Metal Hydride Batteries and Lithium-Ion Batteries | ACS Sustainable Chemistry & Engineering

Ni+H2SO4=H2+Ni2(SO4)3 Balanced Equation||Nickel+Sulphuric acid=Hydrogen+Nickel sulphate Balanced Equ - YouTube

How to balance Ni+H2SO4=Ni2(SO4)3+H2|Chemical equation Ni+H2SO4=Ni2(SO4)3+H2| Ni+H2SO4=Ni2(SO4)3+H2 - YouTube